Review of Your Previous Knowledge
Question 1.
Can you mention some articles that are made up of metals?
Answer:
Jewellery, conducting wires, utensils, etc.
Question 2.
Do metals exist in nature in the form same as that we use in our daily life?
Answer:
No, they exist as ores and minerals.
Question 3.
Have you ever heard the words like ore, mineral and metallurgy?
Answer:
Yes, these words are related to extraction of metals.
Question 4.
Do you know how these metals are obtained?
Answer:
These metals are generally extracted from their ores.
Improve Your Learning
Question 1.
List three metals that are found in nature as oxide ores.
Answer:
The three metals that are found in nature as oxide ores are
- Bauxite (Al2O3 2H2O)
- Haematite (Fe2O3)
- Zincite (ZnO).
Question 2.
List three metals that are found in nature in uncombined form.
Answer:
The three metals that are found in nature in uncombined form are
- Gold
- Silver
- Platinum.
Question 3.
Write a note on dressing of ore in metallurgy.
(OR)
What is concentration of Ore? List various physical methods that are used to enrich the ore.
Answer:
- Ores that are mined from the earth are usually contaminated with large amount of impurities such as soil and sand, etc.
- Concentration or dressing means, simply getting rid of as much of the unwanted rocky material as possible from the ore. The impurities like sand and clay are called gangue.
The physical methods adopted in dressing of the ore or enriching the ore depends upon the difference between physical properties of ore and gangue.
Methods of dressing or concentration of the ore:
1. Hand picking :
If the ore particles and the impurities are different in one of the properties like colour, size, etc. using that property, the ore particles are handpicked separating them from other impurities.
2. Washing:
- We use washing method with water to separate dust from rice, dal and vegetable fruits, etc.
- Ore particles are crushed and kept on a slopy surface. They are washed with controlled flow of water. Less densive impurities are carried away by water flow, leaving the more densive ore particles behind.
3. Froth flotation:
This method is mainly useful for sulphide ores. The ore with impurities is finely powdered and kept in water taken in a flotation cell. Air under pressure is blown to produce froth in water. Froth so produced, takes the ore particles to the surface whereas impurities settle at the bottom. Froth is separated and washed to get ore particles.
4. Magnetic separation:
If the ore or impurity, one of them is magnetic substance and the other is non-magnetic substance, they are separated using electromagnets.
Question 4.
What is an ore? On what basis a mineral is chosen as an ore?
Answer:
Ore :
A mineral from which a metal can be extracted economically and conveniently is called ore.
A mineral is chosen as an ore if the mineral is economical and profitable to extract.
Example:
Aluminium is the common metal in the Earth’s crust in all sorts of minerals. It is economically feasible and profitable to extract from bauxite which contains 50-70% of aluminium oxide.
Question 5.
Write the names of any two ores of iron.
Answer:
The names of two ores of iron :
- Haematite (Fe2O3)
- Magnetite (Fe3O4).
Question 6.
How do metals occur in nature ? Give examples to any two types of minerals.
Answer:
- The earth’s crust is the major source of metals.
- Sea water also contains some soluble salts such as sodium chloride and magnesium chloride etc.
- Some metals like gold (Au), silver (Ag) and copper (Cu) are available in nature in free state as they are least reactive.
- Other metals are found in nature in the combined form due to their more reactivity.
- The elements or compounds of the metals which occur in nature in the earth’s crust are called minerals.
Examples :
Minerals in oxide form :
Bauxite, Zincite, Magnetite, etc.
Minerals in sulphide form :
Copper iron pyretes, Galena, etc.
Question 7.
Write short notes on froth flotation process.
(OR)
Which method is useful for concentration of sulphide ore? Explain the method.
Answer:
Froth Floatation process :
- This method is mainly useful for sulphide ores which have no wetting property whereas impurities get wetted.
- The ore with impurities is finely powdered and kept in water taken in a floatation cell.
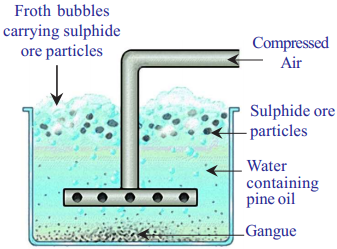
Froth floatation process for the concentration of sulphide ores
- Air under pressure is blown to produce froth in water.
- Froth so produced takes the ore particles to the surface whereas impurities settle at the bottom.
- Froth is separated and washed to get ore particles.
Question 8.
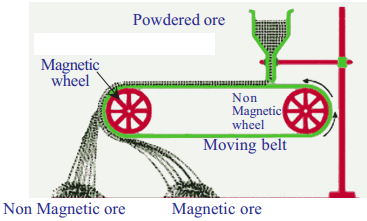
When do we use magnetic separation method for concentration of an ore? Explain with an example.
(OR)
Write the name of the method we use to separate the ore or impurity in which one of them is magnetic substance. Draw a neat diagram indicating the method.
Answer:
If the ore or impurity, one of them is magnetic substance and the other non-magnetic substance they are separated using electromagnets.
Ex :
Iron from iron ore (Fe3O4) is separated from its impurity by passing through a magnetic field. The field attracts magnetic ore (Iron) and repels the non-magnetic impurities.
Question 9.
Write short notes on each of the following :
i) Roasting
ii) Calcination
iii) Smelting
Answer:
i) Roasting :
Roasting is a pyrochemical process in which the ore is heated in the presence of oxygen or air, below its melting point. Generally, reverberatory furnace is used for roasting.
Ex:
Zinc blende on heating with oxygen in reverberatory furnace forms zinc oxide as solid and liberating sulphur dioxide as gas.
2Zns(s)+ 3O2(g)→ 2ZnO(s)+ 2SO2(g)
ii) Calcination :
Calcination is a pyrochemical process in which the ore is heated in the absence of air. The ore gets generally decomposed in this process.
Ex: MgCO3(s)→ MgO(s)+ CO2(g)
iii)Smelting:
Smelting is a pyrochemical process, in which the ore is mixed with flux and fuel, then is strongly heated.
Question 10.
What Is the difference between roasting and calcination? Give one example for each.
(OR)
Roasting and Calcination are the methods to extract crude metals from ores. What is the difference between Roasting and Calcination?
Answer:
| Roasting |
Calcination |
| 1. Roasting is a pyrochemical process in which the ore is heated in the presence of air below its melting point. |
1. Calcination is a pyrochemical process in which the ore is heated in the absence of air. |
| 2. It is an oxidation reaction. |
2. It is a decomposition reaction. |
| 3. It requires oxygen. |
3. It doesn’t require oxygen. |
| 4. It is suitable to sulphide ores. |
4. It is suitable to carbonate ores. |
| 5. Ex : 2ZnS + 3O2→ 2ZnO + 2SO2 |
5. Ex : CaCO3→ CaO + CO2 |
Question 11.
Define the terms:
i) gangue
ii) slag.
Answer:
i) Gangue:
The impurity present in the ore is called gangue.
(or)
Unwanted impurity associated with ore.
ii) Slag:
The impurities found from molten metal during poling process of refining are called slag.
Question 12.
Magnesium is an active metal if it occurs as a chloride in nature, which method of reduction is suitable for its extraction?
Answer:
- The method of reduction which is useful for chloride of magnesium which is active is electrolytic reduction.
- Fused MgCl2is electrolysed with steel cathode (-) and graphite anode (+). The metal (Mg) will be deposited at cathode and chlorine gas liberates at the anode.
At cathode : Mg2++ 2 e-→ Mg
At anode : 2 Cl–→ Cl2+ 2e–
Question 13.
Mention two methods which produce very pure metals.
Answer:
Methods which produce very pure metals are :
- Electrolytic Reduction
- Smelting.
(OR)
- Distillation
- Poling.
Question 14.
Which method do you suggest for extraction of high reactivity metals? Why?
Answer:
1.The only method which is suitable for extraction of high reactivity metals is electrolysis of their fused compounds.
2. Other methods are not suitable due to following reasons :
a) Simple reduction methods like heating with C, CO, etc. to reduce the ores of these metals are not suitable because the temperature required for the reduction is too high and more expensive.
b) Electrolysis of their aqueous solutions are also not preferable because water in the solution would be discharged at the cathode in preference to the metal ions.
Question 15.
Suggest an experiment to prove that the presence of air and water is essential for corrosion. Explain the procedure.
(OR)
Write the experimental procedure to prove that water and air are essential for rusting of iron articles.
(OR)
How can you prove that the presence of air and humid are essential for corrosion?
(OR)
Explain in brief, an experiment to prove that the presence of air and water are essential for corrosion.
Write the precautions to be taken in the experiment to show air and water are essential for rusting iron articles and also write the experimental procedure.
Answer:
Aim :
To prove that the presence of air and water is essential for corrosion or for rusting of iron articles.
Apparatus :
3 boiled test tubes, 3 corks, boiled distilled water, anhydrous calcium chloride, clean iron nails.
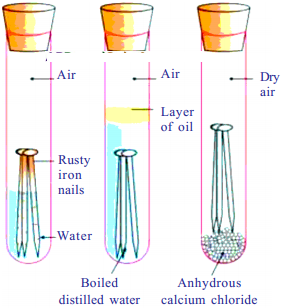
Procedure :
- Take three test tubes and place clean iron nails in each of them.
- Label these test tubes A, B and C. Pour some water in test tube A and cork it.
- Pour boiled distilled water in test tube B, add about 1 ml of oil and cork it. The oil will float on water and prevent the air from dissolving in the water.
- Put some anhydrous calcium chloride in test tube C and cork it. Anhydrous calcium chloride will absorb the moisture.
- Leave these test tubes for a few days and then observe.
- We will observe that iron nails rust in test tube A, but they do not rust in test tubes B and C.
Observation :
- In test tube A, the nails are exposed to air and water. Hence, the nails rusted.
- In test tube B, the nails are exposed only to water, but not to air, because the oil float on water and prevent the air from dissolving in the water. Hence, the nails are not rusted.
- In test tube C, the nails are exposed to dry air, because anhydrous calcium chloride will absorb the moisture, if any, from the air. Hence, the nails are not rusted.
Conclusion :
From the above experiment we can prove that air and water are essential for corrosion.
Question 16.
Collect information about extraction of metals of low reactivity silver, platinum and gold and prepare a report.
(OR)
Prepare a report with the collected information about extraction of metals of low reactivity silver, platinum and gold.
Answer:
Extraction of Silver:
- Silver can be extracted from Ag2S by using displacement from aqueous solution.
- Ag2S is dissolved in KCN solution to get dicyanoargentate (I) ions.
- From these ions Ag is precipitated by treating with Zinc dust powder.
Ag2S + 4 CN–→ 2 [Ag(CN)2]–+ S2-
2[Ag(CN)2]–(aq)+ Zn(s)→ [Zn(CN)4]2-(aq)+ 2Ag(s)
Extraction of Gold :
- Gold is extracted from gold ore like electrum. Impurities are separated from the gold by treating gold ore with a weak cyanide solution.
- Zinc is added and a chemical reaction takes place which separates the gold from ore.
- Pure gold is removed from the solution with a filter press.
Extraction of Platinum:
- The extraction of platinum from ore is a complex process and includes milling the ore, a froth flotation process, and smelting at high temperatures.
- This removes the base metals, notably iron and sulphur and concentrate platinum.
Question 17.
Draw the diagram showing
i) Froth flotation
ii) Magnetic separation.
(OR)
Draw a neat diagram and label the parts that shows froth floatation process for the concentration of sulphide ore.
Answer:
i)

ii)

Question 18.
Draw a neat diagram of reverberatory furnace and label it neatly.
(OR)
Draw a neat labelled diagram of a reverberatory furnace.
Answer:
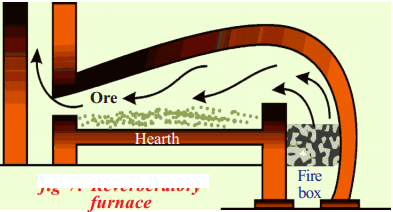
Question 19.
What is activity series? How does it help in extraction of metals?
Answer:
Activity Series :
We can arrange metals in descending order of their reactivity. This series of writing metals is called activity series.
Uses of activity series the extraction of metals :
- Activity series is extremely useful in extraction of metals because we can judge the nature of metal and how it exists.
- High reactive metals like K, Na, Ca, Mg and Al are so reactive that they are never found in nature in free state.
- The moderate reactive metals like Zn, Fe, Pb, etc. are found in the earth’s crust mainly as oxides, sulphides and carbonates.
- The least reactive metals like Au, Ag, Pt are found even in free state in nature.
Question 20.
What is thermite process? Mention its applications in daily life.
Answer:
Thermite Process :
- Thermite process is the chemical reaction which takes place between metal oxides and aluminium.
- When highly reactive metals such as sodium, calcium, aluminium, etc. are used as reducing agents, they displace metals of lower reactivity from the compound.
- This reaction is highly exothermic. The amount of heat evolved is so high that the metals can he directly converted into molten state.
Applications in daily life :
- The reaction if Iron (III) oxide (Fe203) with aluminium is used to join railing of railway tracks or cracked machine parts.
2 Al + Fe2O3→ Al2O3+ 2 Fe + Heat.
- And also used for joining of cracked metal utensils in the house.
Question 21.
Where do we use hand picking and washing methods in our daily life ? Give examples.
How do you correlate these examples with enrichment of ore?
Answer:
Daily life examples for hand picking:
- Separating mud particles from rice is an example for hand picking because the colour and size of these two are different.
- Similarly, the ore particles and the impurities are different in one of the properties like colour, size, etc. are separated by hand picking.
Daily life examples for washing :
- We can clean some vegetables like potatoes by controlled flow of water. Less densive impurities are carried away by the flow leaving the more densive potatoes.
- Similarly, ores are washed with controlled flow of water. Less densive impurities i are carried away by water flow, leaving the more densive ore particles behind.